Alcohols Instructional Video For 10th Higher Ed Lesson Planet
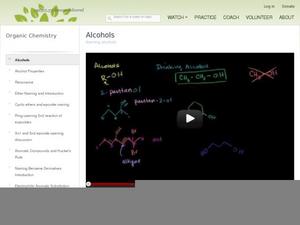
Alcohols Instructional Video For 10th Higher Ed Lesson Planet Alcohols may be considered as organic derivatives of water (h2o) in which a hydrogen atom has been replaced by an alkyl group. examples include ethanol, methanol, and isopropyl alcohol. Alcohols are referred to as allylic or benzylic if the hydroxyl group is bonded to an allylic carbon atom (adjacent to a c=c double bond) or a benzylic carbon atom (next to a benzene ring), respectively.

Organic Chemistry Alcohols Lesson Plan For 9th 12th Grade Lesson Planet Alcohol boiling point, solubility, flammability: most of the common alcohols are colourless liquids at room temperature. methyl alcohol, ethyl alcohol, and isopropyl alcohol are free flowing liquids with fruity odours. The most common reactions of alcohols can be classified as oxidation, dehydration, substitution, esterification, and reactions of alkoxides. alcohols may be oxidized to give ketones, aldehydes, and carboxylic acids. Many alcohols occur naturally and are valuable intermediates in the synthesis of other compounds because of the characteristic chemical reactions of the hydroxyl group. One of the most common members of the alcohol family of organic compounds and the first commercial synthetic alcohol.
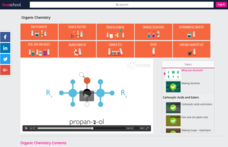
What Are Alcohols Instructional Video For 10th Higher Ed Lesson Planet Many alcohols occur naturally and are valuable intermediates in the synthesis of other compounds because of the characteristic chemical reactions of the hydroxyl group. One of the most common members of the alcohol family of organic compounds and the first commercial synthetic alcohol. Alcoholic beverage, any fermented liquor, such as wine, beer, or distilled spirits, that contains ethyl alcohol, or ethanol, as an intoxicating agent. alcoholic beverages are fermented from the sugars in fruits, berries, grains, and such other ingredients as plant saps, tubers, honey, and milk. Ethanol, a member of a class of organic compounds that are given the general name alcohols. ethanol is an important industrial chemical; it is used as a solvent, in the synthesis of other organic chemicals, and as an additive to gasoline. Ethanol is an excellent motor fuel with a high octane rating and low emissions; however, it should be used in a fuel system designed to withstand the alcohol’s tendency to dissolve plastic parts. solutions of 10 percent ethanol in gasoline (gasohol) can be used in most cars without any adjustments. Alcohol consumption alcohol and society: the origin of alcoholic beverages is lost in the mists of prehistory. fermentation can occur in any mashed sugar containing food—such as grapes, grains, berries, or honey—left exposed in warm air. yeasts from the air act on the sugar, converting it to alcohol and carbon dioxide. alcoholic beverages were thus probably discovered accidentally by.
Comments are closed.