Difference Between Acetone And Styrofoam Difference Between

Difference Between Acetone And Styrofoam Difference Between Acetone Vs Styrofoam Explore the key differences between acetone and styrofoam, including their properties, uses, and applications in various industries. What is the difference between acetone and styrofoam? compare acetone vs styrofoam in tabular form, in points, and more. check out definitions, examples, images, and more.
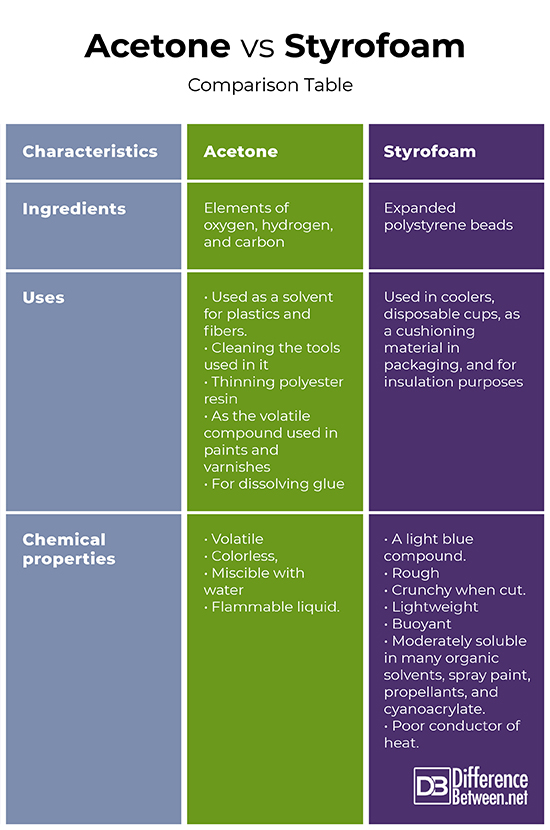
Print Difference Between Print Acetone is an organic solvent commonly used as a nail polish remover, while styrofoam is a polystyrene foam used for insulation and packaging. acetone can dissolve styrofoam due to its ability to break down polystyrene molecules. Acetone is miscible with water and many other organic solvents, whereas styrofoam is only moderately soluble in organic solvents. acetone is mainly used as a cleansing agent, while styrofoam is used mostly in coolers, in packaging as a cushioning material and for insulation. Styrofoam is a trademarked brand of closed cell extruded polystyrene foam (xps), commonly called "blue board", manufactured as foam continuous building insulation board used in walls, roofs, and foundations as thermal insulation and water barrier. Acetone is naturally produced by the body as a product of metabolism. polystyrene, on the other hand, is a crystal clear, glossy and relatively brittle synthetic polymer which usually softens when heated beyond its glass transition temperature.

Acetone Vs Styrofoam Difference And Comparison Styrofoam is a trademarked brand of closed cell extruded polystyrene foam (xps), commonly called "blue board", manufactured as foam continuous building insulation board used in walls, roofs, and foundations as thermal insulation and water barrier. Acetone is naturally produced by the body as a product of metabolism. polystyrene, on the other hand, is a crystal clear, glossy and relatively brittle synthetic polymer which usually softens when heated beyond its glass transition temperature. While styrofoam does not decompose quickly or easily, acetone makes it seem to disappear in seconds. this is because acetone is a solvent that breaks down styrofoam. Acetone, or propanone, is an organic compound with the formula (ch3)2co. it is the simplest and smallest ketone. it is a colourless, highly volatile and flammable liquid with a characteristic pungent odour. In this article, we will delve into the interaction between acetone and styrofoam, exploring the potential hazards, the science behind the reaction, and safety precautions. This video demonstrates the physical reaction of dissolving styrofoam (polystyrene) into acetone (nail polish remover) and gives some explanation as to how the volume of styrofoam changes so that a seemingly large piece can fit into a small space.
Difference Between Acetone And Water Difference Between Acetone Vs Water While styrofoam does not decompose quickly or easily, acetone makes it seem to disappear in seconds. this is because acetone is a solvent that breaks down styrofoam. Acetone, or propanone, is an organic compound with the formula (ch3)2co. it is the simplest and smallest ketone. it is a colourless, highly volatile and flammable liquid with a characteristic pungent odour. In this article, we will delve into the interaction between acetone and styrofoam, exploring the potential hazards, the science behind the reaction, and safety precautions. This video demonstrates the physical reaction of dissolving styrofoam (polystyrene) into acetone (nail polish remover) and gives some explanation as to how the volume of styrofoam changes so that a seemingly large piece can fit into a small space.
Comments are closed.