Difference Between Acetone And Water Difference Between Acetone Vs Water
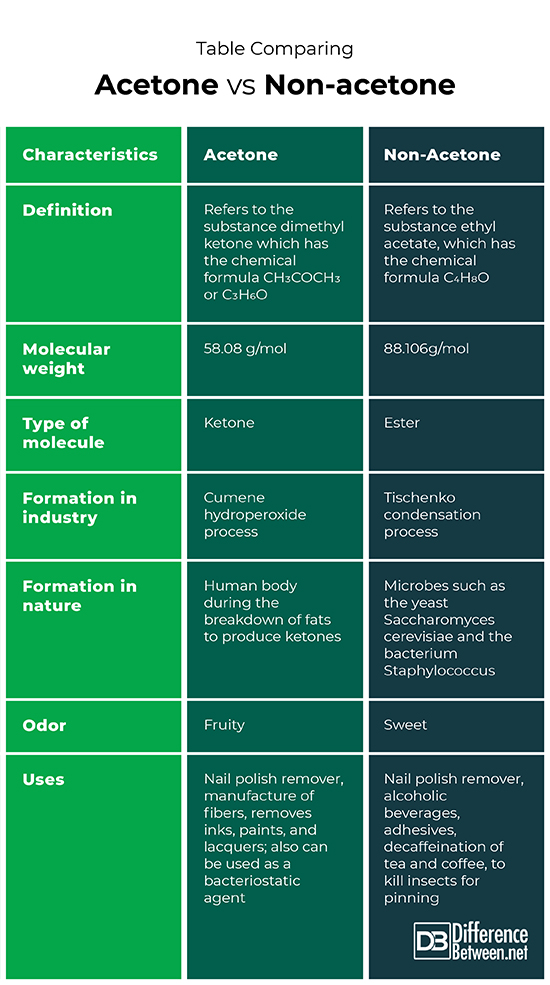
Difference Between Acetone Vs Non Acetone Difference Between Explore the key differences between acetone and water, including their chemical properties, uses, and characteristics. The main difference between acetone and water is that acetone is the simplest ketone with a specific odour, on the other hand, water is an inorganic compound, which is odourless.

Difference Between Acetone And Non Acetone Difference Betweenz Acetone is an organic solvent, while water is a universal solvent and an inorganic compound. acetone evaporates quickly and is highly flammable, whereas water has a higher boiling point and is not flammable. In this blog post, we’re going to dive into the chemistry of both solvents to give you an in depth look at the difference between acetone and water. find out for yourself which one is better for cleaning surfaces and why it’s so important to understand their unique differences!. The simple answer is: yes, acetone is miscible in water. when acetone and water are mixed, they readily form a clear, homogeneous solution regardless of the proportions used. no phase separation occurs; you won't see two distinct layers forming. this directly demonstrates their miscibility. Acetone dissolves completely when mixed with water. in this reaction, acetone is the solute and water is the solvent. when acetone mixes with water, hydrogen bonds form between these compounds. these bonds will keep acetone dissolved completely in water, resulting in a homogeneous solution.

Acetone Vs Acetate What S The Difference The simple answer is: yes, acetone is miscible in water. when acetone and water are mixed, they readily form a clear, homogeneous solution regardless of the proportions used. no phase separation occurs; you won't see two distinct layers forming. this directly demonstrates their miscibility. Acetone dissolves completely when mixed with water. in this reaction, acetone is the solute and water is the solvent. when acetone mixes with water, hydrogen bonds form between these compounds. these bonds will keep acetone dissolved completely in water, resulting in a homogeneous solution. Water (chemical formula h2o) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of earth’s hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). The solvent dissolves the acetone, creating two distinct layers: one rich in acetone and the other in water. the layers are then separated, and the solvent can be removed from the acetone through distillation or evaporation. Water and acetone are indeed miscible, but acetone is significantly less polar, and nacl is not soluble in acetone. hence when acetone and water mix, the decreased solubility of the mixture overpowers the fact that there is more solvent, and hence some of the dissolved salt will precipitate out.

Acetone Vs Acetylene What S The Difference Water (chemical formula h2o) is an inorganic, transparent, tasteless, odorless, and nearly colorless chemical substance, which is the main constituent of earth’s hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). The solvent dissolves the acetone, creating two distinct layers: one rich in acetone and the other in water. the layers are then separated, and the solvent can be removed from the acetone through distillation or evaporation. Water and acetone are indeed miscible, but acetone is significantly less polar, and nacl is not soluble in acetone. hence when acetone and water mix, the decreased solubility of the mixture overpowers the fact that there is more solvent, and hence some of the dissolved salt will precipitate out.
Comments are closed.