Difference Between Validation Calibration And Qualification In Pharma

Difference Between Validation Calibration And Qualification In Pharma There is often confusion surrounding the terms validation, calibration, and qualification within the pharmaceutical industry. let’s explore their differences with explanatory examples. What is the difference between validation, qualification, and calibration? calibration ensures measurement accuracy, qualification verifies equipment readiness, and validation ensures process consistency.

Difference Between Validation Calibration And Qualification In Pharma A lot of pharmaceutical professionals are having a big confusion among calibration, validation and qualification, hence i am trying to wash out the confusion. i think it will make a clear image of these three concepts and their definitions. In summary, calibration focuses on ensuring the accuracy of measuring instruments, validation ensures the reliability and accuracy of methods and systems, and qualification verifies the suitability and compliance of equipment, facilities, and systems used in the pharmaceutical industry. Core concepts like calibration, validation, and qualification are vital for maintaining compliance, meeting good manufacturing practices (gmp), and ensuring the consistency of manufacturing processes. these three processes, though interconnected, have distinct purposes and applications in pharmaceutical operations. Calibration focuses on accuracy and precision, while qualification focuses on functionality and performance . calibration can be done in house or by a third party service provider, while qualification may require involvement from regulatory agencies or independent auditors.

Difference Between Validation Calibration And Qualification In Pharma Core concepts like calibration, validation, and qualification are vital for maintaining compliance, meeting good manufacturing practices (gmp), and ensuring the consistency of manufacturing processes. these three processes, though interconnected, have distinct purposes and applications in pharmaceutical operations. Calibration focuses on accuracy and precision, while qualification focuses on functionality and performance . calibration can be done in house or by a third party service provider, while qualification may require involvement from regulatory agencies or independent auditors. In short, regular calibration allows pharmaceutical companies to have confidence in their results which they can record, monitor and control. you can read also: difference between calibration and validation. To ensure the safety, efficacy, and high quality drugs or medical devices, qualification and validation activities are performed with the help of good documentation practices as part of the regulatory requirements. in simple words, these activities ascertain whether the system is designed as needed and perform as intended. Understanding the differences between qualification and validation is crucial for anyone in the pharmaceutical industry. qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Both qualification and validation are integral to ensuring that pharmaceutical products are consistently produced and meet required quality standards. however, understanding the differences between the two is crucial for regulatory compliance and maintaining product quality.
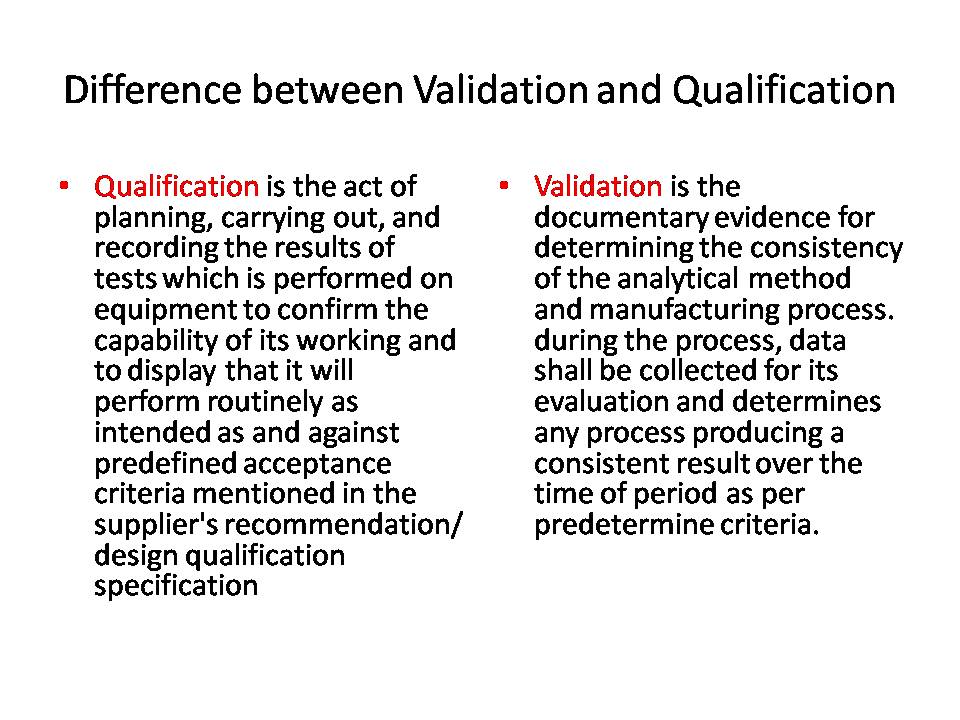
Difference Between Validation Calibration And Qualification In Pharma In short, regular calibration allows pharmaceutical companies to have confidence in their results which they can record, monitor and control. you can read also: difference between calibration and validation. To ensure the safety, efficacy, and high quality drugs or medical devices, qualification and validation activities are performed with the help of good documentation practices as part of the regulatory requirements. in simple words, these activities ascertain whether the system is designed as needed and perform as intended. Understanding the differences between qualification and validation is crucial for anyone in the pharmaceutical industry. qualification is the process of verifying that a piece of equipment or a particular item is fit for its intended purpose. Both qualification and validation are integral to ensuring that pharmaceutical products are consistently produced and meet required quality standards. however, understanding the differences between the two is crucial for regulatory compliance and maintaining product quality.
Comments are closed.