Drug Testing Instructions Reading Results 12 Panel Now
Drug Testing Instructions Reading Results 12 Panel Now The use of psychoactive drugs without medical supervision is associated with significant health risks and can lead to the development of drug use disorders. drug use disorders, particularly when untreated, increase morbidity and mortality risks for individuals, can trigger substantial suffering and lead to impairment in personal, family, social, educational, occupational or other important. About who drug information who drug information is a quarterly journal providing an overview of topics relating to medicines development and regulation which is targeted to a wide audience of health professionals and policy makers. launched in 1987, who drug information communicates the latest international news and trends to regulatory agencies, academic and training institutions, researchers.
Drug Testing Instructions Reading Results 12 Panel Now A new report from the world health organization (who) highlights that 2.6 million deaths per year were attributable to alcohol consumption, accounting for 4.7% of all deaths, and 0.6 million deaths to psychoactive drug use. notably, 2 million of alcohol and 0.4 million of drug attributable deaths were among men. The unit works globally to improve health and well being of populations by articulating, promoting, supporting and monitoring evidence informed policies, strategies and interventions to reduce the burden associated with alcohol, drugs and addictive behaviours. The world health organization (who) today released its updated bacterial priority pathogens list (bppl) 2024, featuring 15 families of antibiotic resistant bacteria grouped into critical, high and medium categories for prioritization. Who announces development of updated guidelines for the psychosocially assisted pharmacological treatment of opioid dependence and community management of opioid overdosein 2022, approximately 60 million people globally engaged in non medical opioid use, including the use of drugs like heroin, morphine, codeine, fentanyl, methadone, tramadol, and other similar substances. their regular non.

Ensuring Accurate Drug Testing 12 Panel Now The world health organization (who) today released its updated bacterial priority pathogens list (bppl) 2024, featuring 15 families of antibiotic resistant bacteria grouped into critical, high and medium categories for prioritization. Who announces development of updated guidelines for the psychosocially assisted pharmacological treatment of opioid dependence and community management of opioid overdosein 2022, approximately 60 million people globally engaged in non medical opioid use, including the use of drugs like heroin, morphine, codeine, fentanyl, methadone, tramadol, and other similar substances. their regular non. 1. introduction and background manufacturers should ensure that the products that they manufacture are safe, efective and of the quality required for their intended use. systems should be in place to ensure that pharmaceutical products are produced according to validated processes and to defined procedures. manufacturing processes should be shown to be capable of consistently manufacturing. Following recommendations by the world health organization (who), the united nations commission on narcotic drugs (cnd) has decided to place five new psychoactive substances and one medicine under international control. The who repository of national essential medicines lists (nemls) serves as a comprehensive repository of national essential medicines lists, national formularies, and national standard treatment guidelines. these documents play an important role in guiding the procurement and supply of medicines in the public sector, informing reimbursement schemes, directing medicine donations, and shaping. The mission of the who international clinical trials registry platform is to ensure that a complete view of research is accessible to all those involved in health care decision making. this will improve research transparency and will ultimately strengthen the validity and value of the scientific evidence base. registration of all interventional trials is a scientific, ethical and moral.

12 Panel Now On Linkedin 12paneldrugtest 12paneldrugscreentest 12panelnow 12panelurinedrugtest 1. introduction and background manufacturers should ensure that the products that they manufacture are safe, efective and of the quality required for their intended use. systems should be in place to ensure that pharmaceutical products are produced according to validated processes and to defined procedures. manufacturing processes should be shown to be capable of consistently manufacturing. Following recommendations by the world health organization (who), the united nations commission on narcotic drugs (cnd) has decided to place five new psychoactive substances and one medicine under international control. The who repository of national essential medicines lists (nemls) serves as a comprehensive repository of national essential medicines lists, national formularies, and national standard treatment guidelines. these documents play an important role in guiding the procurement and supply of medicines in the public sector, informing reimbursement schemes, directing medicine donations, and shaping. The mission of the who international clinical trials registry platform is to ensure that a complete view of research is accessible to all those involved in health care decision making. this will improve research transparency and will ultimately strengthen the validity and value of the scientific evidence base. registration of all interventional trials is a scientific, ethical and moral.

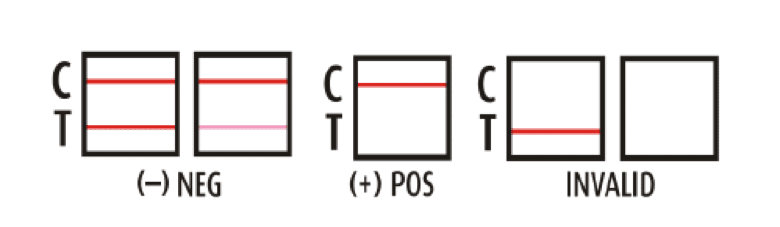
How To Read A 12 Panel Drug Test 12 Panel Now The who repository of national essential medicines lists (nemls) serves as a comprehensive repository of national essential medicines lists, national formularies, and national standard treatment guidelines. these documents play an important role in guiding the procurement and supply of medicines in the public sector, informing reimbursement schemes, directing medicine donations, and shaping. The mission of the who international clinical trials registry platform is to ensure that a complete view of research is accessible to all those involved in health care decision making. this will improve research transparency and will ultimately strengthen the validity and value of the scientific evidence base. registration of all interventional trials is a scientific, ethical and moral.
Comments are closed.