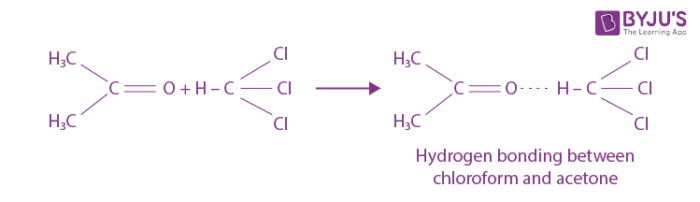
Enthalpy Change During Interaction Between Acetone And Chloroform Chemistry Practicals Class 12
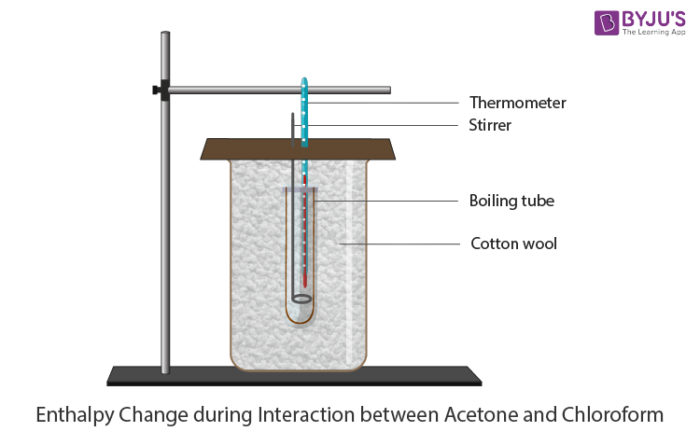
Enthalpy Change During Interaction Between Acetone And Chloroform Chemistry Practicals Class 12 An enthalpy change describes the change in enthalpy observed in the constituents of a thermodynamic system when undergoing a transformation or chemical reaction. Enthalpy is an energy like property or state function—it has the dimensions of energy (and is thus measured in units of joules or ergs), and its value is determined entirely by the temperature, pressure, and composition of the system and not by its history.
Enthalpy Change During Interaction Between Acetone And Chloroform Chemistry Practicals Class 12 Enthalpy is a state function of a thermodynamic system and depends on other state functions. mathematically, it is the sum of the internal energy and the product of the pressure and volume of the system. In thermodynamics, the enthalpy is the measure of energy in a thermodynamic system. it is the thermodynamic quantity equivalent to the total heat content of a system. the enthalpy is defined to be the sum of the internal energy e plus the product of the pressure p and volume v. Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: h = u pv (1) (1) h = u p v. when a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal to the change in enthalpy. Learn what enthalpy is, its formula, significance in thermodynamics, and real world applications in engineering and science.

Chemistry Practical Class 12 Viva Questions On Determination Of Enthalpy Change During Enthalpy (h h) is the sum of the internal energy (u u) and the product of pressure and volume (pv p v) given by the equation: h = u pv (1) (1) h = u p v. when a process occurs at constant pressure, the heat evolved (either released or absorbed) is equal to the change in enthalpy. Learn what enthalpy is, its formula, significance in thermodynamics, and real world applications in engineering and science. Enthalpy is the measurement of energy in a thermodynamic system. the quantity of enthalpy equals to the total content of heat of a system, equivalent to the system’s internal energy plus the product of volume and pressure. Enthalpy is the measurement of heat or energy in the thermodynamic system. it is the most fundamental concept in the branch of thermodynamics. it is denoted by the symbol h. in other words, we can say, enthalpy is the total heat of the system. let's know more about enthalpy in detail below. The enthalpy change of a reaction depends on the physical states of the reactants and products, so these must be shown. for example, when 1 mole of hydrogen gas and mole of oxygen gas change to 1 mole of liquid water at the same temperature and pressure, 286 kj of heat are released. if gaseous water forms, only 242 kj of heat are released. Enthalpy is a central factor in thermodynamics. it is the heat content of a system. the heat that passes into or out of the system during a reaction is the enthalpy change.
Comments are closed.