Experiment 7 Colours In Chemistry A Pdf Redox Chemistry

Experiment 7 Redox Titration Pdf Titration Chemistry The document describes an experiment involving the colors of transition metal complexes. it explains that the colors observed are due to electrons being excited to higher energy states when compounds absorb visible light. In this article, we describe a vivid colour changing demonstration to illustrate a chain of redox reactions, whereby electrons are transferred between different compounds and ions. the activity is suitable as a teacher demonstration, or older students could carry out the experiment themselves.

C Ch 07 Redox Reactions And Volumetric Analysis Pdf Redox Chemistry Pink brown cu strip was added to colourless zn2 solution. the solution turns blue (cu2 ) and a silvery grey deposit of zn is formed. silvery grey fe strip was added to colourless h solution. bubbles are produced (h2) and the solution remains colourless (fe2 ). First, while reacting to the various complex compounds, observe the colours formed. secondly, to measure the shift in the oxidation states in order to prepare the normal solution of vanadium. methods two portions of the experiment were performed. To understand this laboratory experiment better, let us calculate the theoretical values of e0 cell for the pb(s)| pb2 (aq)||cu 2 (aq)|cu(s) cell at different temperatures. The color of the transmitted light is called the complementary color of that of the absorbed light, in fact the color of complex or in other words opposite. they are simply colors which are directly opposite each other in the color wheel such as blue with green.
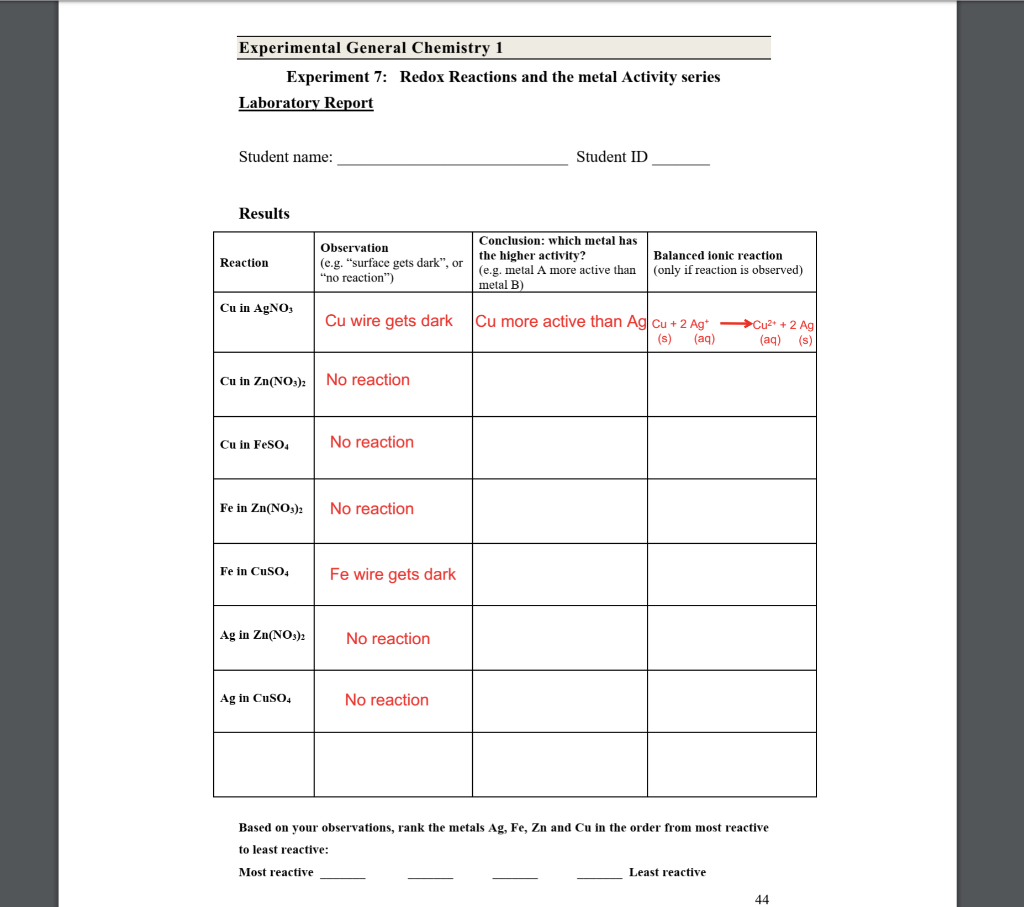
Solved Experimental General Chemistry 1 Experiment 7 Redox Chegg To understand this laboratory experiment better, let us calculate the theoretical values of e0 cell for the pb(s)| pb2 (aq)||cu 2 (aq)|cu(s) cell at different temperatures. The color of the transmitted light is called the complementary color of that of the absorbed light, in fact the color of complex or in other words opposite. they are simply colors which are directly opposite each other in the color wheel such as blue with green. In this exercise, you perform simple redox reactions and, on the basis of your observations, deduce the relative oxidising abilities of a number of oxidising agents. In this experiment you will use experimental evidence and additional information to write a potential series for some cations, halogens, and hydrogen ion. the reactions you will carry out to determine a potential series for metals and hydrogen will be done in the separate wells of a spot plate. In this section, you will perform a redox titration that involves the reaction of potassium permanganate (kmno4) with iron (ii) ammonium sulfate hexahydrate, fe(nh4)2(so4)2•6h2o. If a compound absorbs radiation from the visible part of the electromagnetic spectrum, then the colour observed for the compound is the complementary colour to that absorbed.

Redox Chemistry Google Docs Pdf Redox Chemistry Purpose The Purpose Of This Lab For The In this exercise, you perform simple redox reactions and, on the basis of your observations, deduce the relative oxidising abilities of a number of oxidising agents. In this experiment you will use experimental evidence and additional information to write a potential series for some cations, halogens, and hydrogen ion. the reactions you will carry out to determine a potential series for metals and hydrogen will be done in the separate wells of a spot plate. In this section, you will perform a redox titration that involves the reaction of potassium permanganate (kmno4) with iron (ii) ammonium sulfate hexahydrate, fe(nh4)2(so4)2•6h2o. If a compound absorbs radiation from the visible part of the electromagnetic spectrum, then the colour observed for the compound is the complementary colour to that absorbed.

Experiment 7 Colours In Chemistry A Pdf Redox Chemistry In this section, you will perform a redox titration that involves the reaction of potassium permanganate (kmno4) with iron (ii) ammonium sulfate hexahydrate, fe(nh4)2(so4)2•6h2o. If a compound absorbs radiation from the visible part of the electromagnetic spectrum, then the colour observed for the compound is the complementary colour to that absorbed.
Comments are closed.