H2o Ai Review Best Machine Learning Platform In 2025 Honest Review
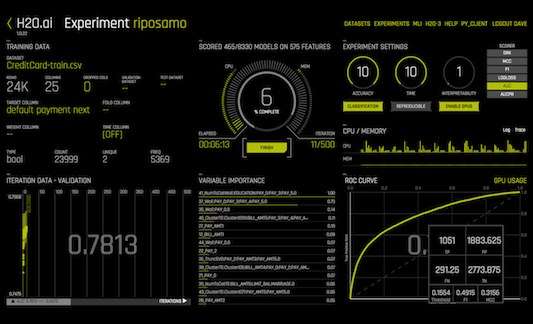
H2o Ai Powerful Machine Learning Ai Data Science Platform Tech Pilot %yield=67.5% the percent yield can be calculated by: %yield=("actual yield") ("theoretical yield")xx100% the actual yield of oxygen is given and it is 10.2g. we will need to find the theoretical yield. the decomposition reaction of water can be written as: 2h 2o(l) >2h 2(g) o 2(g) to find the theoretical yield of oxygen we will use dimensional analysis: ?g o 2=underbrace(17.0gh 2oxx(1molh 2o. So molar mass of the gas is =30g (mol) it is also given that on burning 1.5g of gas produces 4.4g of co2 and 2.7g of h2o so burning of 30g of gas produces ((4.4xx30) 1.5)g of co2and((2.7.xx30) 1.5)g of h2o i.e.burning of 30g of gas produces 88g of co2 and 54g of h2o i.e.burning of 1 mol of gas produces 88 44mol of co2and54 18molof h2o i.e.
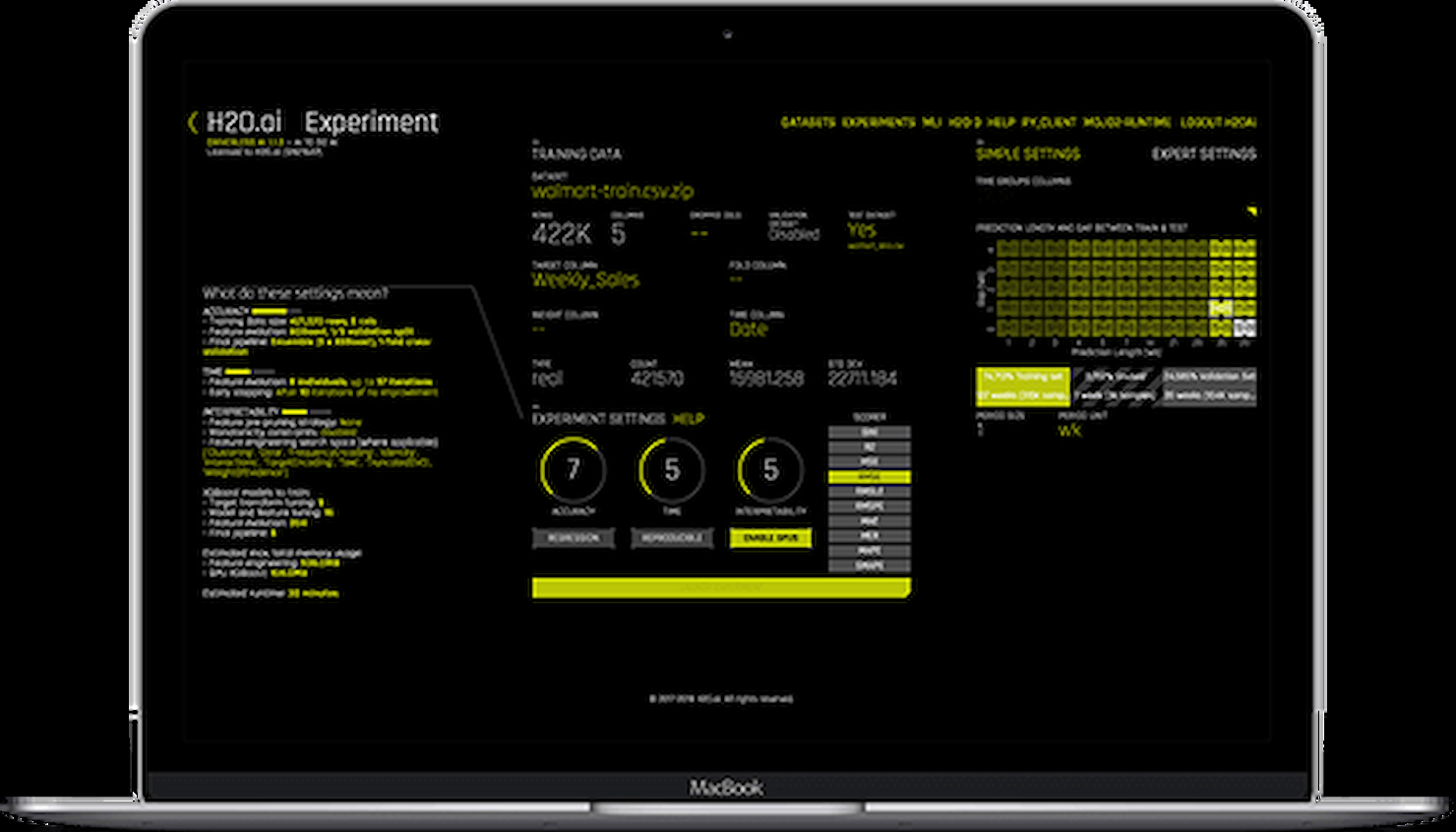
Guide To The Top 20 Machine Learning Cloud Platforms In 2025 The Cto Club None. hydrogen atoms only have one electron to share with the oxygen atom, so there are no lone pairs of electrons around either hydrogen atom. however, the oxygen atom has two lone pairs of electrons. Gas in solid form, the molecules are closest together. in liquid, they are touching, but somewhat apart. in the form of gas, they are free; bouncing off each other. therefore, water molecules are farthest apart in the state of gas. Changes in state are physical changes. let's take water as an example. h2o. in the gaseous state, we sometimes call it steam or water vapor; it's chemical formula is still h2o. in the liquid state and in the solid state (ice), the chemical formula is h2o. in a physical change, the original substance is still there it may be in a different state or shape, but there are no new substances. Which of the following compounds exhibits only dispersion and dipole dipole intermolecular interactions ? a) hbr b) co2 c) h2o d) n2 question #91f53 how come an atom cannot have a permanent dipole moment? question #dbcca why are homonuclear diatomic molecules non polar?.
Ai Startup H2o Ai Launches Driverless Ai An Automated Machine Learning Platform Tech Startups Changes in state are physical changes. let's take water as an example. h2o. in the gaseous state, we sometimes call it steam or water vapor; it's chemical formula is still h2o. in the liquid state and in the solid state (ice), the chemical formula is h2o. in a physical change, the original substance is still there it may be in a different state or shape, but there are no new substances. Which of the following compounds exhibits only dispersion and dipole dipole intermolecular interactions ? a) hbr b) co2 c) h2o d) n2 question #91f53 how come an atom cannot have a permanent dipole moment? question #dbcca why are homonuclear diatomic molecules non polar?. Irreversibly. this is sometimes exploited by chemists. the carbanionic nucleophile reacts with the electrophilic proton on the water molecule: r mgx h 2o rarr r h mgxohdarr the reaction is irreversible, but it does have some synthetic utility if you want to label an hydorcarbyl chain with an isotope of hydrogen, ""^2h or ""^3h, simply by using heavy or tritiated water. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. what is the molar percentage of co in the mixture in the cylinder?. The clue lies in the name itself. double displacement reactions occur when two ionic compounds react, or when an ionic compound reacts with an acid. it only occurs in liquids. there are 5 types of double displacement reaction, as shown below: salt salt = insoluble salt salt acid carbonate = salt h2o co2 acid base = salt h2o ammonium hydroxide = salt h2o nh3 acid sulfite. If density is 0.92 g cm–3 at a given temperature, then what is the number of h2o molecules per unit cell? a 9.380 mol sample of nitrogen gas is maintained in a 0.8198 l container at 301.8 k. what is the pressure in atm calculated using the van der waals' equation for n2 gas under these conditions?.
20 Best Ai Driven Machine Learning Platforms Artificial Intelligence Tools Review Irreversibly. this is sometimes exploited by chemists. the carbanionic nucleophile reacts with the electrophilic proton on the water molecule: r mgx h 2o rarr r h mgxohdarr the reaction is irreversible, but it does have some synthetic utility if you want to label an hydorcarbyl chain with an isotope of hydrogen, ""^2h or ""^3h, simply by using heavy or tritiated water. A cylinder of volume 1m3 is filled with 1kg of co and 100g of h2o. what is the molar percentage of co in the mixture in the cylinder?. The clue lies in the name itself. double displacement reactions occur when two ionic compounds react, or when an ionic compound reacts with an acid. it only occurs in liquids. there are 5 types of double displacement reaction, as shown below: salt salt = insoluble salt salt acid carbonate = salt h2o co2 acid base = salt h2o ammonium hydroxide = salt h2o nh3 acid sulfite. If density is 0.92 g cm–3 at a given temperature, then what is the number of h2o molecules per unit cell? a 9.380 mol sample of nitrogen gas is maintained in a 0.8198 l container at 301.8 k. what is the pressure in atm calculated using the van der waals' equation for n2 gas under these conditions?.
Comments are closed.