Interchangeability And Switching Study Designs For Biosimilars
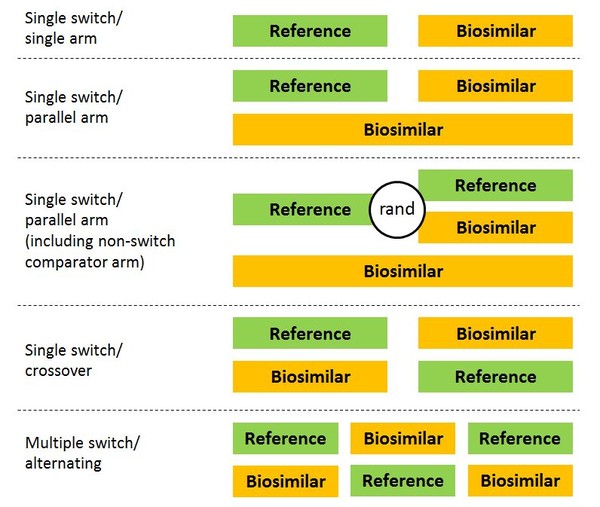
Interchangeability And Switching Study Designs For Biosimilars This draft guidance describes considerations regarding a switching study or studies intended to support a demonstration that a biological product is interchangeable with a reference. Clinical studies that support interchangeability should be designed primarily to evaluate if clinical performance is altered by multiple switching between a reference product and its biosimilar and whether such switching will result in differences in pharmacokinetics or immunogenicity profiles.
Interchangeability Switching Of Biosimilars How to establish interchangeability and selection of possible designs of switching studies for biosimilars were discussed by dr hans ebbers of the medicines evaluation board (meb cbg) in the netherlands at the european stakeholder event on biosimilars in brussels, belgium [3]. To be granted this interchangeable designation, the us food and drug administration (fda) specified that postmarketing pharmacovigilance data should be combined with data from at least one prospective, controlled multiple switching study in patients with a disease for which the biosimilar is licensed. 2 this switching study should consist of a. Starting with drug classes where the evidence of clinical equivalence supporting interchangeability is strongest, fda should consider a pathway to market that does not require switching studies on top of requirements to demonstrate biosimilarity. On june 20, 2024, the u.s. food and drug administration (fda) published updated draft guidance titled “consideration for demonstrating interchangeability with a reference product: update.”.

Interchangeability Between Biologics And Biosimilars Cvs Specialty Starting with drug classes where the evidence of clinical equivalence supporting interchangeability is strongest, fda should consider a pathway to market that does not require switching studies on top of requirements to demonstrate biosimilarity. On june 20, 2024, the u.s. food and drug administration (fda) published updated draft guidance titled “consideration for demonstrating interchangeability with a reference product: update.”. In this article, we examine the statistical properties, analyses, and sample size requirements of these switching study designs. we also investigate the relative efficiencies of these switching designs compared with the complete n of 1 trial design. In its draft guidance, the fda recommends a switching study or studies, in which patients are switched between the biosimilar and reference product and monitored for any clinical meaningful difference, to support a demonstration of interchangeability. On june 20, 2024, fda issued a draft guidance on biosimilar interchangeability that revises the need for switching studies to demonstrate a biosimilar is interchangeable. Part 3 of this series for global biosimilars week, penned by dracey poore, director of biosimilars at cardinal health, explores the critical topic of interchangeability, examining its role in shaping biosimilar adoption and the broader implications for accessibility.
Comments are closed.