Lever Rule Phase Diagram
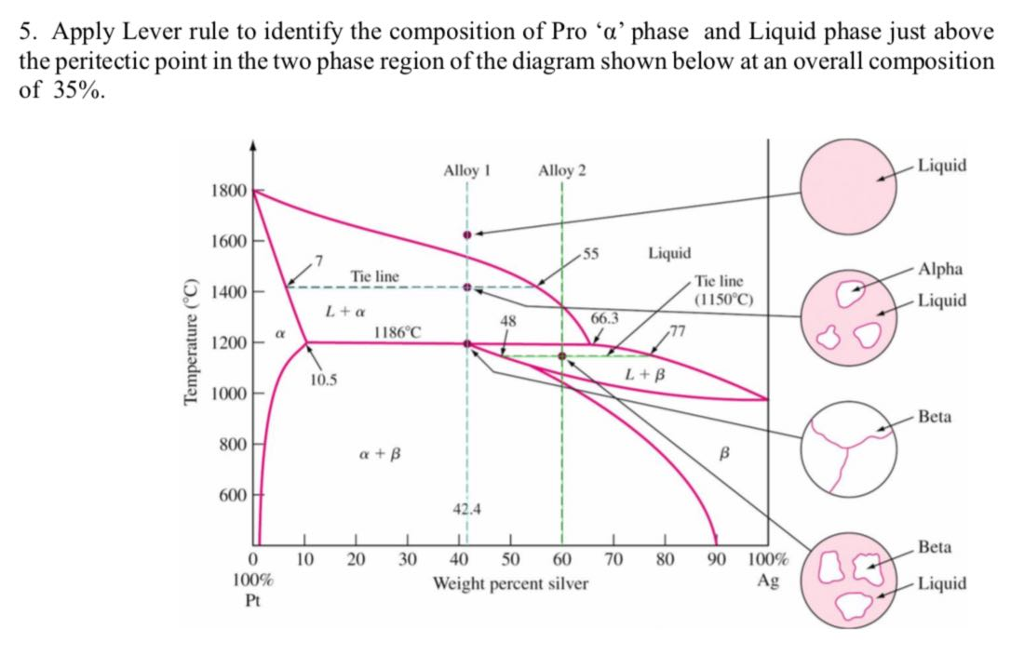
Lever Rule Phase Diagram So, at point e on you diagram above, there is one specific temperature and composition at which $\delta$, $\gamma$, and $\epsilon$ are in equilibrium. go up in temperature, and it wants to be all $\delta$; come down in temperature and you enter the two phase $\gamma$ $\delta$ region with the relative abundance from the lever rule. I have the relevant ternary phase diagram, but i am unsure exactly about interpreting it because of the "doubled" stoichiometry of the sodium and potassium salts on the plot. i posit that along the drawn in red line, there is a 50 50 molar mixture of ca(no3)2 and (kno3)2.
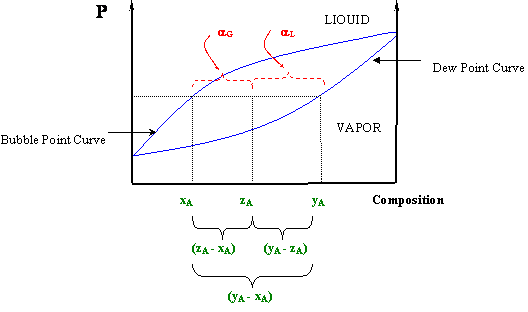
Lever Rule Phase Diagram The compositions of the both the liquid and the phase vapor are not independent of temperature, given the constraint that the liquid is in equilibrium with the vapor. the composition of the liquid phase will be given by the lower curve. and the composition of the vapor phase is given by the upper curve. temperature as the single degree of freedom. Using the lever rule, the amount of liquid in the 2 phase region is given by $$\frac{18 0}{31 0}=0.58$$ hence for the amount of solid in the same region we get $$1 0.58=0.42$$ since the overall sum of the liquid and solid in the two phase region is 1. The three phase (eutectic) equilibrium phase compositions are not along a line in the phase diagram. each of the three phases is instead at a single point (specifically, where the isotherm touches the field having that phase), which dovetails with zero degrees of freedom. Eutectic phase diagram and lever rule. 5. boundary lines in phase diagrams and the lever rule. 14.

Lever Rule Phase Diagram Examples The three phase (eutectic) equilibrium phase compositions are not along a line in the phase diagram. each of the three phases is instead at a single point (specifically, where the isotherm touches the field having that phase), which dovetails with zero degrees of freedom. Eutectic phase diagram and lever rule. 5. boundary lines in phase diagrams and the lever rule. 14. Stack exchange network. stack exchange network consists of 183 q&a communities including stack overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Eutectic phase diagram and lever rule. 6. having trouble understanding the iron carbon phase diagram. 1. Likewise, at the azeotropic point, in addition to the vapor phase, the liquid exists in immiscible layers of liquids rich in x and y, respectively. therefore, in both cases there are three phases, leading to conditional degree of freedom (or variance) of zero, where the word "conditional" refers to a specific pressure and "zero" means a fixed. If you follow the line for say for 12 $^\circ$ c, from the degree notation there is at first one phases, a gas phase. now when you reach the dotted curve there are two phases a gas phase and a liquid phase which are joined by a horizontal tie line. the whole thing is a lot easier to figure out with a more traditional phase diagram.

Lever Rule Phase Diagram Examples Stack exchange network. stack exchange network consists of 183 q&a communities including stack overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Eutectic phase diagram and lever rule. 6. having trouble understanding the iron carbon phase diagram. 1. Likewise, at the azeotropic point, in addition to the vapor phase, the liquid exists in immiscible layers of liquids rich in x and y, respectively. therefore, in both cases there are three phases, leading to conditional degree of freedom (or variance) of zero, where the word "conditional" refers to a specific pressure and "zero" means a fixed. If you follow the line for say for 12 $^\circ$ c, from the degree notation there is at first one phases, a gas phase. now when you reach the dotted curve there are two phases a gas phase and a liquid phase which are joined by a horizontal tie line. the whole thing is a lot easier to figure out with a more traditional phase diagram.
Comments are closed.