Oxidation Of Ammonia Pharmacist Blogger Lab Chemistry Laboratory

Laboratory Preparation Of Ammonia Chemistry Lab Sajha Notes #lab #laboratory #labrador #chemistry #chemical #ammonia #burn thanku for watching. In this video, we showcase the catalytic oxidation of ammonia utilising chromium (iii) oxide as the catalyst. paul cook guides you through each step of the experiment, providing insights into the chemical reactions and the underlying principles of catalysis.

Laboratory Preparation Of Ammonia Chemistry Lab Sajha Notes Ammonia oxidation is a fundamental core process in the global biogeochemical nitrogen cycle. oxidation of ammonia (nh 3) to nitrite (no 2 −) is the first and rate limiting step in nitrification and is carried out by distinct groups of microorganisms. The direct oxidation of ammonia to nitric acid has been known for a considerable time, as shown by the experiments of milner, who, in 1788, passed a mixture of ammonia and air over heated manganese dioxide with the production of oxides of nitrogen. Ammonia 1. laboratory preparation: 2nh 4 cl ca(oh) 2 → cacl 2 2h 2 o 2nh 3↑ i. when ammonium salts are heated with strong alkali, ammonia gas is produced. [strong alkali and produced a weaker alkali] ii. but ammonium nitrate and nitrite cannot be used as they produce n 2 o and n 2 when heated. nh 4 no 3 → n 2 o 2h 2 o nh 4 no 2. Ammonia oxidation experiments were conducted at high pressure (30 bar and 100 bar) and temperatures of 450–900 k in oxidizing and stoichiometric conditions. the data were interpreted in terms of a detailed chemical kinetic model, developed for high pressure conditions.

Laboratory Preparation Of Ammonia Chemistry Lab Sajha Notes Ammonia 1. laboratory preparation: 2nh 4 cl ca(oh) 2 → cacl 2 2h 2 o 2nh 3↑ i. when ammonium salts are heated with strong alkali, ammonia gas is produced. [strong alkali and produced a weaker alkali] ii. but ammonium nitrate and nitrite cannot be used as they produce n 2 o and n 2 when heated. nh 4 no 3 → n 2 o 2h 2 o nh 4 no 2. Ammonia oxidation experiments were conducted at high pressure (30 bar and 100 bar) and temperatures of 450–900 k in oxidizing and stoichiometric conditions. the data were interpreted in terms of a detailed chemical kinetic model, developed for high pressure conditions. So about ammonia, it will start to burst heat at room temperature it this includes the glass she considered putting the ammonia piece. this basis would make ammonia likely ignite at room temperatures of 1204 degrees fahrenheit with vapor or 1800 degrees celsius to 650 degrees celsius. Electrochemical ammonia oxidation reaction (aor) presents a promising avenue for realizing sustainable nitrogen cycling in various energy and environmental applications. however, sluggish catalytic activity, catalyst poisoning effects, and low stability pose significant challenges. Share your videos with friends, family, and the world. There are two main practical methods for obtaining ammonia – one of them in the laboratory, the other in industry: n₂ 2h₂ = 2nh₃ (reversible reaction). this method of obtaining ammonia is called the haber reaction.
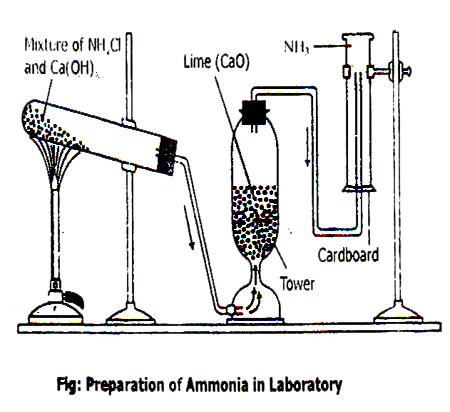
Lab Preparation Of Ammonia Qs Study So about ammonia, it will start to burst heat at room temperature it this includes the glass she considered putting the ammonia piece. this basis would make ammonia likely ignite at room temperatures of 1204 degrees fahrenheit with vapor or 1800 degrees celsius to 650 degrees celsius. Electrochemical ammonia oxidation reaction (aor) presents a promising avenue for realizing sustainable nitrogen cycling in various energy and environmental applications. however, sluggish catalytic activity, catalyst poisoning effects, and low stability pose significant challenges. Share your videos with friends, family, and the world. There are two main practical methods for obtaining ammonia – one of them in the laboratory, the other in industry: n₂ 2h₂ = 2nh₃ (reversible reaction). this method of obtaining ammonia is called the haber reaction.

Chemistry Pdf Ammonia Chemical Reactions Share your videos with friends, family, and the world. There are two main practical methods for obtaining ammonia – one of them in the laboratory, the other in industry: n₂ 2h₂ = 2nh₃ (reversible reaction). this method of obtaining ammonia is called the haber reaction.
Comments are closed.