Planar Autonomous Systems Of Ordinary Differential Equations Solution

Planar Autonomous Systems Of Ordinary Differential Equations Solution Otherwise, its structure allows it to be planar. even though the molecule will have a structure that allows for it to exist in a planar conformation, there may be some many that do not persist in a planar conformation due to steric effects, or complex three dimensional geometries. The molecule $\ce { [pdcl4]^2 }$ is diamagnetic, which indicates a square planar geometry as all eight d electrons are paired in the lower energy orbitals. however, $\ce { [nicl4]^2 }$ is also $\mathr.

Planar Autonomous Systems Of Ordinary Differential Equations Solution In this case, both the planar representation and the conformational analysis lead to the same conclusion about the chirality of cis 1,2 dimethylcyclohexane (though for different reasons). will the planar representation and conformational analysis always lead to the same conclusion about chirality?. How can one predict whether a given complex ion will be square planar or tetrahedral when its coordination number is 4 using crystal field theory? is it possible to theoretically predict this?. For a trigonal planar complex (e.g. [agclx3]x2− [a g c l x 3] x 2): according to this image (source): the x2 −y2 x 2 y 2 and xy x y orbitals are on the same energy level. however, assuming that one of the cl c l ligands lies on the y y axis (or the x x axis), wouldn't the x2 −y2 x 2 y 2 orbital be higher in energy? thanks in advance. 1 it is because of the fact that square planar complexes are formed by much strong ligands with d8 metal cation of 3d series transition metals cation and 4d or 5d series transition metal cation with either weak or strong ligands. the very strong ligands and 4d or 5d series transition metal cations are responsible for higher crystal field.
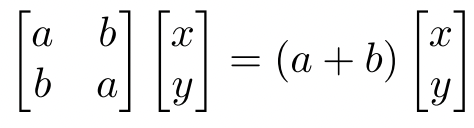
Planar Autonomous Systems Of Ordinary Differential Equations Solution For a trigonal planar complex (e.g. [agclx3]x2− [a g c l x 3] x 2): according to this image (source): the x2 −y2 x 2 y 2 and xy x y orbitals are on the same energy level. however, assuming that one of the cl c l ligands lies on the y y axis (or the x x axis), wouldn't the x2 −y2 x 2 y 2 orbital be higher in energy? thanks in advance. 1 it is because of the fact that square planar complexes are formed by much strong ligands with d8 metal cation of 3d series transition metals cation and 4d or 5d series transition metal cation with either weak or strong ligands. the very strong ligands and 4d or 5d series transition metal cations are responsible for higher crystal field. Does this mean that all p orbitals have planar nodes and in d, only d 2 d z 2 has conical node while others have planar node. i am clear about radial spherical nodes. thanks. A planar shape evidently is antiaromatic, so cyclooctatetraene either bends into a chair or tub shape. the closest two carbons that are not bonded directly to each other have a distance of ~2.5 angstroms, a sufficient distance where steric factors become significant. Since pph₃ is strong field ligand and, the famous wilkinson's catalyst, which also possess this ligand is square planar, then what makes the above complex tetrahedral?. In the class, i was told that $\\ce{h3c^.}$ has a trigonal planar structure with the unpaired electron in $\\mathrm{2p z}$ orbital. but $\\ce{h3c }$ has a trigonal pyramidal structure. but why does.

Pdf Solution Of Fractional Autonomous Ordinary Differential Equations Does this mean that all p orbitals have planar nodes and in d, only d 2 d z 2 has conical node while others have planar node. i am clear about radial spherical nodes. thanks. A planar shape evidently is antiaromatic, so cyclooctatetraene either bends into a chair or tub shape. the closest two carbons that are not bonded directly to each other have a distance of ~2.5 angstroms, a sufficient distance where steric factors become significant. Since pph₃ is strong field ligand and, the famous wilkinson's catalyst, which also possess this ligand is square planar, then what makes the above complex tetrahedral?. In the class, i was told that $\\ce{h3c^.}$ has a trigonal planar structure with the unpaired electron in $\\mathrm{2p z}$ orbital. but $\\ce{h3c }$ has a trigonal pyramidal structure. but why does.
Equation Autonomous Ordinary And Partial Differential Equations Solved Exam Docsity Since pph₃ is strong field ligand and, the famous wilkinson's catalyst, which also possess this ligand is square planar, then what makes the above complex tetrahedral?. In the class, i was told that $\\ce{h3c^.}$ has a trigonal planar structure with the unpaired electron in $\\mathrm{2p z}$ orbital. but $\\ce{h3c }$ has a trigonal pyramidal structure. but why does.

Machine Learning Methods For Autonomous Ordinary Differential Equations Deepai
Comments are closed.