Process Validation Pdf

Process Validation Pdf Pdf Verification And Validation Food And Drug Administration This guidance outlines the general principles and approaches that fda considers appropriate elements of process validation for the manufacture of human and animal drug and biological products,. A comprehensive document that provides guidance for implementing a lifecycle approach to pharmaceutical process validation. it covers the stages, principles, and best practices of process validation, as well as the regulatory expectations and references.

Process Validation Report Pdf Several firms have asked fda for specific guidance on what fda expects firms to do to assure compliance with the requirements for process validation. this guideline discusses process validation elements and concepts that are considered by fda as acceptable parts of a validation program.the constituents of validation. A presentation on process validation for active pharmaceutical ingredients (apis) based on ich q7 and related guidelines. it covers the general concepts, policy, documentation, qualification, validation program, periodic review and validation of apis used in clinical trials. Recommended stages of process validation, and specific activities for each stage in the product lifecycle. a. general considerations for process validation . in all stages of the product lifecycle, good project management and good archiving that capture scientific knowledge will make the process validation program more effective and efficient. the. Process validation should not be viewed as a one off event. process validation incorporates a lifecycle approach linking product and process development, validation of the commercial manufacturing process and maintenance of the process in a state of control during routine commercial production.

Example Procedure For Process Validation Pdf Verification And Validation Specification Recommended stages of process validation, and specific activities for each stage in the product lifecycle. a. general considerations for process validation . in all stages of the product lifecycle, good project management and good archiving that capture scientific knowledge will make the process validation program more effective and efficient. the. Process validation should not be viewed as a one off event. process validation incorporates a lifecycle approach linking product and process development, validation of the commercial manufacturing process and maintenance of the process in a state of control during routine commercial production. • fda final guidance for industry, process validation: general principles and practices (january 2011). For purposes of this guidance, process validation is defined as the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product. Process verification vs. process validation: what’s the difference? it’s not uncommon to encounter situations in the medical device industry where the terms “process verification” and “process validation” are used interchangeably. Now, the fda defines process validation as the collection and evaluation of data, from the process design stage to validation and throughout production. scientific evidence is to be used to prove that the process is capable of delivering consistently manufactured quality products.
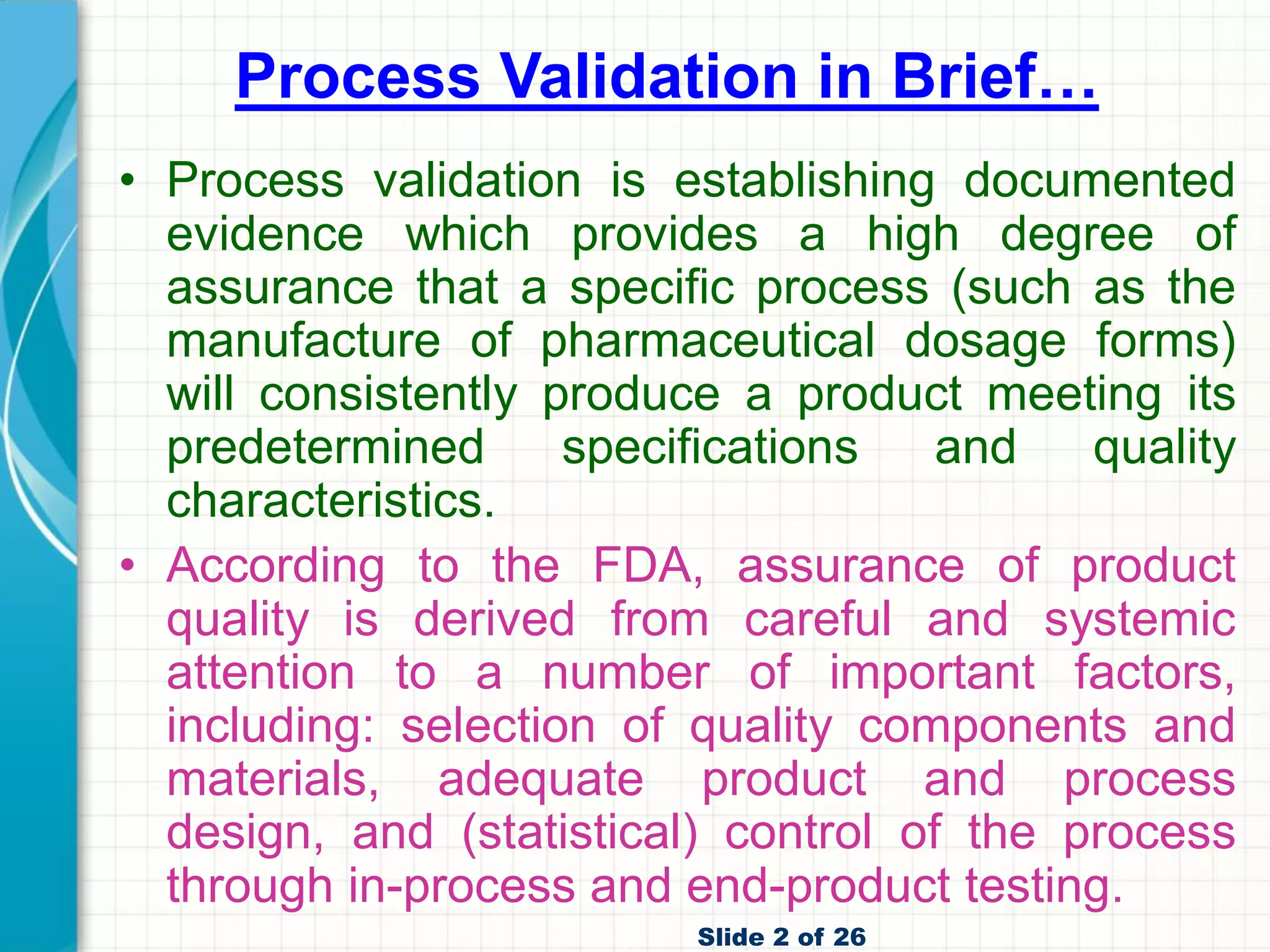
Process Validation Pdf • fda final guidance for industry, process validation: general principles and practices (january 2011). For purposes of this guidance, process validation is defined as the collection and evaluation of data, from the process design stage through commercial production, which establishes scientific evidence that a process is capable of consistently delivering quality product. Process verification vs. process validation: what’s the difference? it’s not uncommon to encounter situations in the medical device industry where the terms “process verification” and “process validation” are used interchangeably. Now, the fda defines process validation as the collection and evaluation of data, from the process design stage to validation and throughout production. scientific evidence is to be used to prove that the process is capable of delivering consistently manufactured quality products.

Process Validation An Essential Process Pdf Download Free Pdf Verification And Validation Process verification vs. process validation: what’s the difference? it’s not uncommon to encounter situations in the medical device industry where the terms “process verification” and “process validation” are used interchangeably. Now, the fda defines process validation as the collection and evaluation of data, from the process design stage to validation and throughout production. scientific evidence is to be used to prove that the process is capable of delivering consistently manufactured quality products.
Comments are closed.