Redox And Non Redox Reactions Experiments Demonstrations C12 1 10 Chemistry Studocu

Redox And Non Redox Reactions Experiments Demonstrations C12 1 10 Chemistry Studocu Redox’s interoperability platform helps providers, payers, health tech vendors, ehrs, and life sciences and pharmaceutical companies power better care with real time data exchange. Terminology "redox" is a portmanteau of "reduction" and "oxidation." the term was first used in a 1928 article by leonor michaelis and louis b. flexner. [6][7] oxidation is a process in which a substance loses electrons. reduction is a process in which a substance gains electrons.

Solution Redox And Non Redox Reactions Experiments And Demonstrations C12 1 10 Studypool Learn what a redox reaction is and how to identify and balance redox reactions. explore oxidation, reduction, and oxidation numbers. What is a redox reaction? a redox reaction is a chemical reaction in which the atoms change their oxidation numbers. some atoms lose electrons and are oxidized – a process known as oxidation. on the other hand, some atoms gain electrons and are reduced – a process known as reduction. An oxidation reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. an oxidation reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox is a shorthand for reduction oxidation, meaning that a redox reaction is one in which both a reduction reaction and an oxidation reaction takes place at once.
O Level Chemistry Tips On Redox Reactions Simplechemconcepts An oxidation reduction (redox) reaction is a type of chemical reaction that involves a transfer of electrons between two species. an oxidation reduction reaction is any chemical reaction in which the oxidation number of a molecule, atom, or ion changes by gaining or losing an electron. Redox is a shorthand for reduction oxidation, meaning that a redox reaction is one in which both a reduction reaction and an oxidation reaction takes place at once. Because of their complementary nature, the oxidation and reduction processes together are referred to as redox reactions. the reactant that brings about the oxidation is called the oxidizing agent, and that reagent is itself reduced by the reducing agent. Redox reactions involve simultaneous reduction and oxidation reactions, during which, electron transfer happens and results in changes in oxidation states. if one substance is oxidised, there must also be a substance reduced. A redox reaction is a reaction where one substance is reduced and another is oxidised. the word redox comes from reduction and oxidation happening at the same time. Redox (shorthand for reduction oxidation) describes all chemical reactions in which atoms have an increase or decrease in oxidation number (oxidation state). [1].
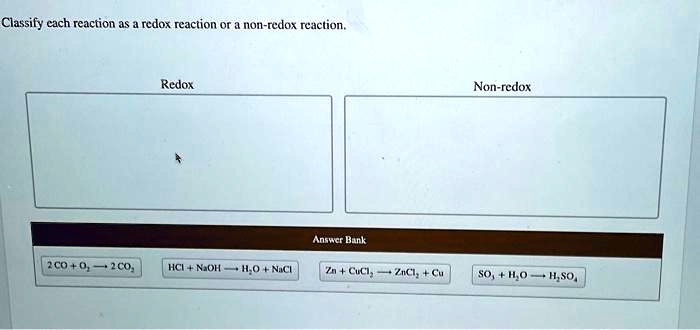
Solved Classify Each Reaction As A Redox Reaction Or A Non Redox Reaction Redox Non Redox Because of their complementary nature, the oxidation and reduction processes together are referred to as redox reactions. the reactant that brings about the oxidation is called the oxidizing agent, and that reagent is itself reduced by the reducing agent. Redox reactions involve simultaneous reduction and oxidation reactions, during which, electron transfer happens and results in changes in oxidation states. if one substance is oxidised, there must also be a substance reduced. A redox reaction is a reaction where one substance is reduced and another is oxidised. the word redox comes from reduction and oxidation happening at the same time. Redox (shorthand for reduction oxidation) describes all chemical reactions in which atoms have an increase or decrease in oxidation number (oxidation state). [1].
Comments are closed.