Security Cloud Native Workshop Workshop Cloud Native Security

Cloud Native Security Part 2 While the pure acid tends to give off white fumes when exposed to air, acid with dissolved nitrogen dioxide gives off reddish brown vapors, leading to the common names "red fuming nitric acid" and "white fuming nitric acid". Is there any minimum quantity or concentration below which one wouldn't have to worry? if a drop were placed in a bathtub full of distilled water and left alone long enough for the water to evaporate, could that drop still pose a hazard?.
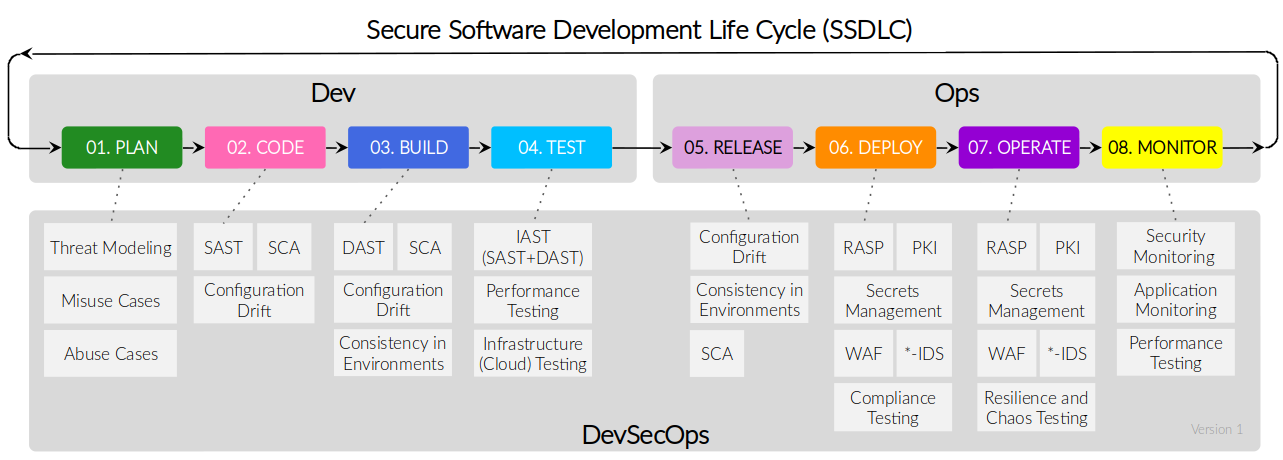
Security Cloud Native Workshop Workshop Cloud Native Security The vaporization of nitric acid has offered extreme difliculties largely because of the corrosive action of the acid on most materials of construction. materials for this purpose have. Fuming nitric acid is concentrated nitric acid that contains dissolved nitrogen dioxide. the density and vapor pressure of such solutions increase with the percentage of nitrogen dioxide present. Nitric acid ranks with hydrochloric acid as being one of the strongest acids. this is due to the high concentration of hydrogen ions in dilute solution resulting from the far reaching dissociation of nitric acid in water. Nitric acid ignites on contact with many organic chemicals and powdered metals, and produces heat and gas (often flammable hydrogen) upon reaction with some other classes of chemicals.

Cloud Security The 4c S Of Cloud Native Security Part 5 Thinktecture Ag Nitric acid ranks with hydrochloric acid as being one of the strongest acids. this is due to the high concentration of hydrogen ions in dilute solution resulting from the far reaching dissociation of nitric acid in water. Nitric acid ignites on contact with many organic chemicals and powdered metals, and produces heat and gas (often flammable hydrogen) upon reaction with some other classes of chemicals. I am dealing with 70% strength nitric acid and in order to see whether this option is safe i need to know how to calculate the evaporation rate of the acid and compare to those allowed by the hse. In the case of iron, concentrated nitric acid will passivate the metal surface whereas diluted nitric acid does not passivate. however, diluted nitric acid may sustain passivity if the metal has been passivated before by other means. Roduce concentrated nitric acid from dilute evaporator overheads. this acid can be recycled to other processes, resulting in reduction of the total amount of acid needed and waste produced. the feed is introduced into the distillation column from feed tanks ts 12 a. Evaporate to dryness, dissolve in a mixture of 4 ml of water and 1 ml of dilute hydrochloric acid (1 in 20), and filter if necessary. wash with two 2 ml portions of water, dilute with water to 10 ml, and add 1 ml of barium chloride ts.

Github Krol3 Workshop Cloud Native Security Workshop About Cloud Native Security I am dealing with 70% strength nitric acid and in order to see whether this option is safe i need to know how to calculate the evaporation rate of the acid and compare to those allowed by the hse. In the case of iron, concentrated nitric acid will passivate the metal surface whereas diluted nitric acid does not passivate. however, diluted nitric acid may sustain passivity if the metal has been passivated before by other means. Roduce concentrated nitric acid from dilute evaporator overheads. this acid can be recycled to other processes, resulting in reduction of the total amount of acid needed and waste produced. the feed is introduced into the distillation column from feed tanks ts 12 a. Evaporate to dryness, dissolve in a mixture of 4 ml of water and 1 ml of dilute hydrochloric acid (1 in 20), and filter if necessary. wash with two 2 ml portions of water, dilute with water to 10 ml, and add 1 ml of barium chloride ts.
Comments are closed.