Solved C I Explain How The Temperature Of A System Can Chegg

Solved C I Explain How The Temperature Of A System Can Chegg Your solution’s ready to go! our expert help has broken down your problem into an easy to learn solution you can count on. see answer. 5 quantity of heat the heat capacity c of a body is the ratio of the amount of heat energy q trans ferred to a body in any process to its corresponding temperature change t .

Solved 1 Explain How The Temperature Of A System Can Be Chegg Consider two closed systems a and b. system a contains 3000 kj of thermal energy at 20°c, whereas system b contains 200 kj of thermal energy at 50°c. now the systems are brought into contact with each other. determine the direction of any heat transfer between the two systems. Solution: the organism can be treated as being in a steady state if we assume that its mass is constant and if we neglect internal motion. matter enters the organism in the form of food, water, and oxygen; waste matter and heat leave the system. Adding heat to a system increases the system's total energy. this gives more kinetic energy to distribute among the particles in the system, increasing the size of the system's phase space and hence its entropy. Learn how to calculate the energy needed to raise the temperature of a system and see examples that walk through sample problems step by step for you to improve your physics knowledge and.
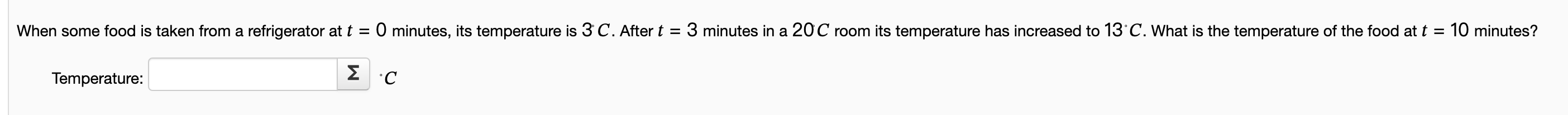
Solved Temperature C Chegg Adding heat to a system increases the system's total energy. this gives more kinetic energy to distribute among the particles in the system, increasing the size of the system's phase space and hence its entropy. Learn how to calculate the energy needed to raise the temperature of a system and see examples that walk through sample problems step by step for you to improve your physics knowledge and. Correct answer: b) the work done by the system is greater than the amount of heat it receives from the surroundings. At chegg we understand how frustrating it can be when you’re stuck on homework questions, and we’re here to help. our extensive question and answer board features hundreds of experts waiting to provide answers to your questions, no matter what the subject. Question: (1) explain how the temperature of a system can be changed without adding or removing heat from the system. (ii) explain how the temperature of a system can remain constant while either adding or removing heat from the system. Result page 3: step by step solutions for physics icse questions from expert tutors over 1:1 instant tutoring sessions. get solutions, concepts, examples or practice problems.
Comments are closed.