Solved For This Two Step Reaction Sequence Identify The Chegg
Solved For This Two Step Reaction Sequence Identify The Chegg This problem has been solved! you'll get a detailed solution from a subject matter expert that helps you learn core concepts. To summarize: the first step involves nucleophilic attack by the grignard reagent on a carbonyl to create an alkoxide, and the second step involves the protonation of that alkoxide to form an alcohol.
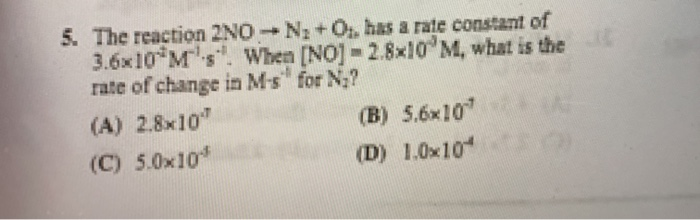
Solved 2 For This Two Step Reaction Sequence Identify The Chegg Draw the products of the two step reaction sequence shown below. use a dash or wedge bond to indicate stereochemistry of substituents on asymmetric centers. ignore inorganic byproducts. Azulenes are soluble in strong aqueous acids because of a reactions with h . which of the structures below would represent the most stable form of protonated azulene?. Find all video solutions for your textbook question solved step by step submitted by pamela r., jul. 21, 2022, 03:31 p.m. Immediate access to step by step solutions: chegg offers detailed, step by step solutions to textbook problems, allowing students to verify their work or understand new problem solving techniques.
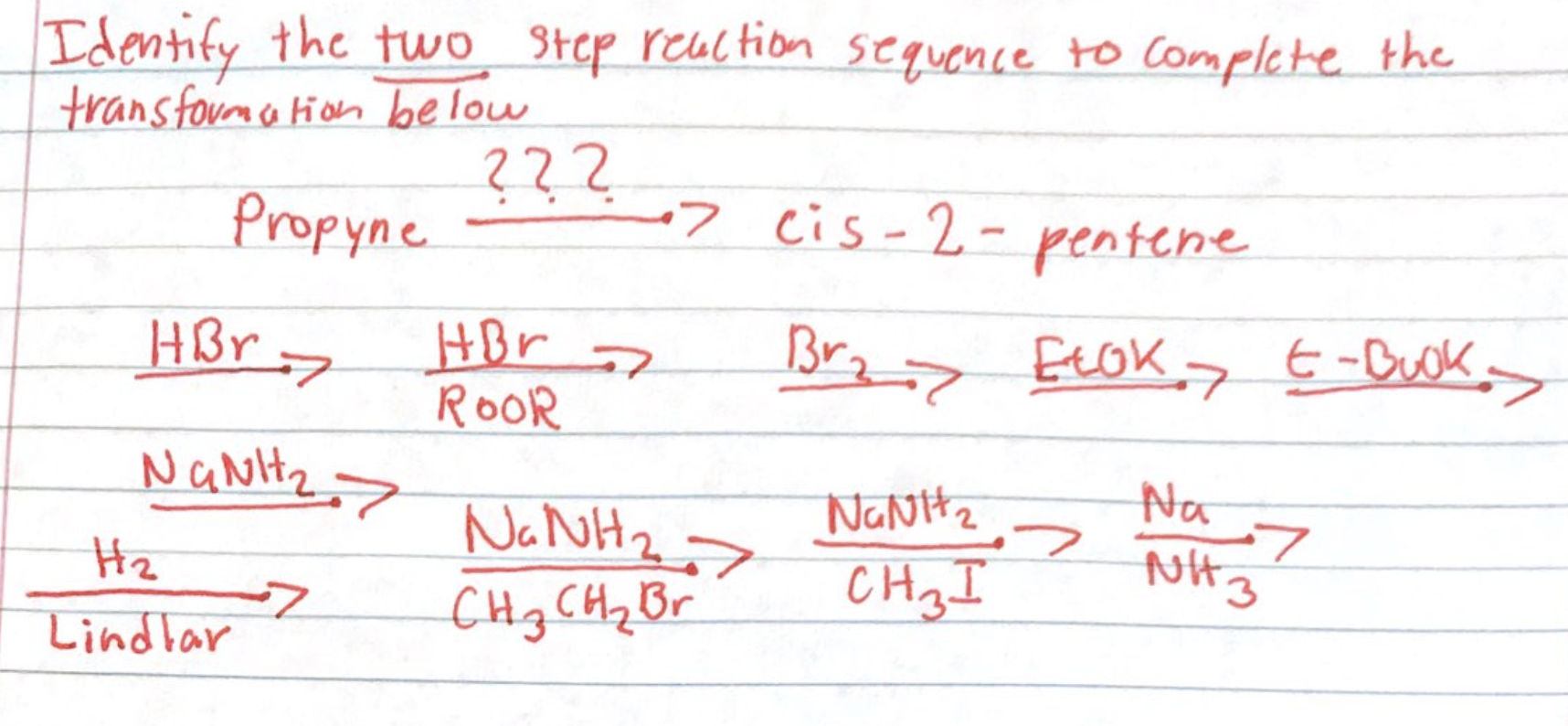
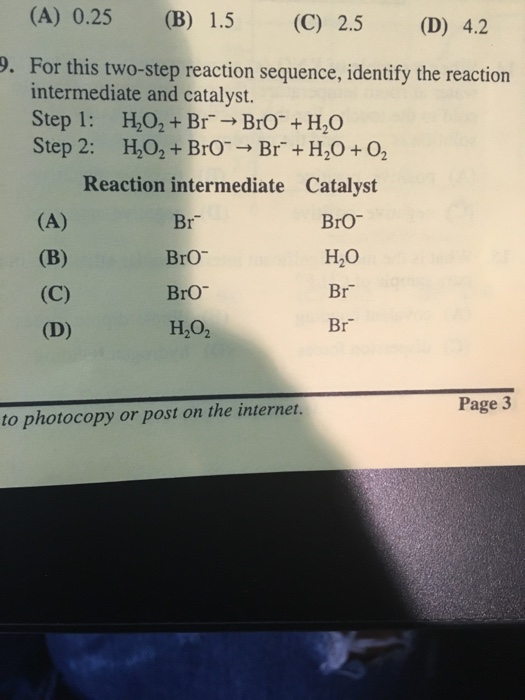
Solved Identify The Two Step Reaction Sequence To Complete Chegg Find all video solutions for your textbook question solved step by step submitted by pamela r., jul. 21, 2022, 03:31 p.m. Immediate access to step by step solutions: chegg offers detailed, step by step solutions to textbook problems, allowing students to verify their work or understand new problem solving techniques. A reaction intermediate is formed in one step of a reaction and used up in a later step. the formation of a reaction intermediate is described in the reaction mechanism. For this two step reaction sequence, identify the reaction intermediate and catalyst step 1: ho, br bro h,o step 2: ho, bro br h0 o reaction intermediate catalyst bao (a) br bro (b) br h,o (c) bro" (d) h,o ba 3 g e 5. A researcher attempts a substitution reaction with two different reagents, water or hydroxide ion, to see which route is better. draw the major, neutral organic compound recovered from each attempted substitution reaction below. Identify the hydroxyl group ( oh) on the starting molecule to understand where the first reagent, pbr3, will react.
Comments are closed.