Solved Part B The Thermodynamic Values From Part A Will Be Chegg
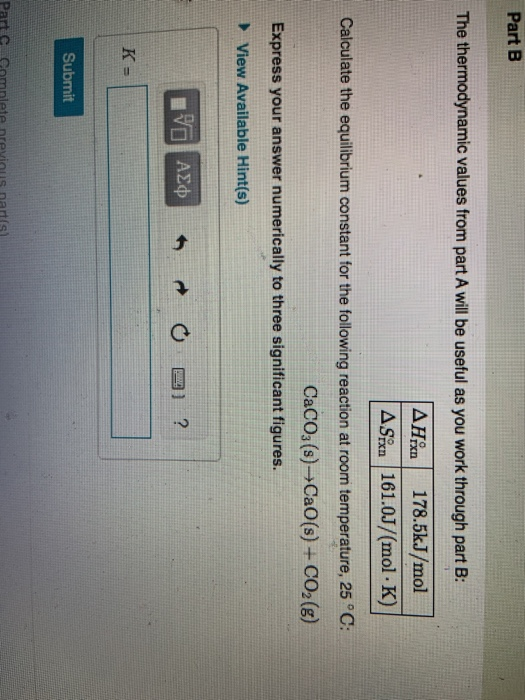
Solved Part B The Thermodynamic Values From Part A Will Be Chegg Part b the thermodynamic values from part a will be useful as you work through part b: ahx 178.5kj mol asixn 161.0j (mol.k) calculate the equilibrium constant for the following reaction at room temperature, 25 °c: caco3(s) cao(s) co2() express your answer numerically to three significant figures. To calculate the equilibrium constant (k) for the reaction at a given temperature, we can use the thermodynamic relation that connects the gibbs free energy change (Δg), the enthalpy change (Δh), the entropy change (Δs), and the temperature (t):.

Solved Part B Ble The Thermodynamic Values From Part A Will Chegg To calculate the equilibrium constant for the reaction b ac o3(s) → b ao(s) c o2(g) at room temperature (25°c), we use the given thermodynamic values: since Δh rxn is given in kj mol and Δs rxn is in j (mol·k), it's necessary to convert Δh rxn∘ to j mol for consistency. Δh rxn∘ = 243.5kj mol ×1000(j kj) = 243500j mol. Solve. the thermodynamic values from part a will be useful as you work through part b: 178.5kj mol astan 161.0j (mol. [solved] the thermodynamic values from part a will be useful as you work through part b: Δh∘rxn 178.5kj mol Δs∘rxn 161.0j (mol⋅k). If the temperature were raised, this would shift to the right and the k c increases for b would be x. this ought to be a plus b. when the temperature goes up, the equilibrium of c plus 5.4 kg….

Solved Part B The Thermodynamic Values From Part A Will Be Chegg [solved] the thermodynamic values from part a will be useful as you work through part b: Δh∘rxn 178.5kj mol Δs∘rxn 161.0j (mol⋅k). If the temperature were raised, this would shift to the right and the k c increases for b would be x. this ought to be a plus b. when the temperature goes up, the equilibrium of c plus 5.4 kg…. Part b. the thermodynamic values from part a will be useful as you work through part b:. Find step by step solutions and answers to thermodynamics : an engineering approach 9781266664489, as well as thousands of textbooks so you can move forward with confidence. The gibbs phase rule d = 2 σ − ϕ gives the dimension of thermodynamic space over which ϕ distinct phases among σ species can coexist. for σ = 1 we have ϕ ≤ 3, since d ≥ 0. Answer to the thermodynamic values from part a will be useful as you work [solved] the thermodynamic values from part a will be useful as you work | course hero[solved] the thermodynamic values from part a will be useful as you work | course hero.
Comments are closed.