Types Of Solutions Learn Definition Different Types In Detail

Solution Definition Types Properties Examples And Faqs 43 Off The following table shows the different types of solutions based on the physical state with examples. solutions can be classified into saturated, unsaturated, and supersaturated types based on the amount of solute dissolved in the solvent under specific conditions, such as temperature and pressure. Learn about different type of solutions, and its types based on the nature of the solvent, concentration, amount of solute added with examples and faqs.
Solution Definition Types Properties Examples And Faqs 43 Off Solutions are combinations of different solutes and a solvent. filtration cannot separate the constituents of a solute. saturated, unsaturated, and supersaturated solutions are all possible. As a result, there are nine different types of solutions depending on the solute and solvent’s physical states. furthermore, it is important to note that a solution is a homogeneous mixture. a true solution does not scatter light. Solutions exist for every possible phase of the solute and the solvent. salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o 2) in a gaseous solvent (n 2). in all cases, however, the overall phase of the solution is the same phase as the solvent. Types of solutions in chemistry: definition, types and importance of types of solutions know all about types of solutions in chemistry.

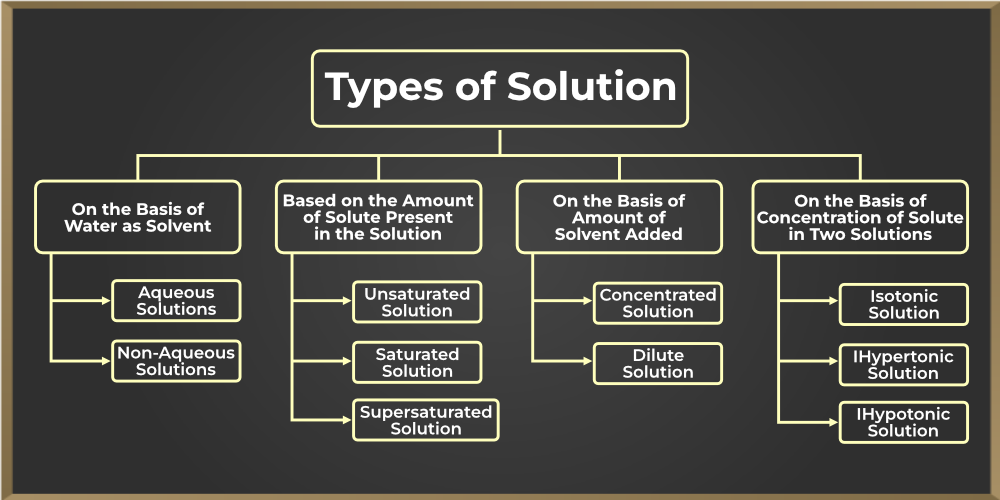
Different Types Of Solutions Homework Minutes Infographics Solutions exist for every possible phase of the solute and the solvent. salt water, for example, is a solution of solid nacl nacl in liquid water, while air is a solution of a gaseous solute (o 2) in a gaseous solvent (n 2). in all cases, however, the overall phase of the solution is the same phase as the solvent. Types of solutions in chemistry: definition, types and importance of types of solutions know all about types of solutions in chemistry. The physical state of a solution—solid, liquid, or gas—is typically the same as that of the solvent, as demonstrated by the examples in table 1. the components of a solution are dispersed on a molecular scale; that is, they consist of a mixture of separated molecules, atoms, and or ions. There are various ways to classify solutions based on different criteria, such as the nature of the solvent, the amount of solvent added, and the concentration of solute in the solution. here are some rephrased statements:. In this article we will discuss aqueous solution, types of solution, homogeneous solution, homogeneous solution examples and get detailed information including the different types, heterogeneous, examples, faqs and more here. Solutions are homogeneous mixtures of two or more substances having components that are uniformly distributed. in a solution, the material in the higher quantity is called the solvent, whereas the substance in the lesser quantity is called the solute.

Solution Chemistry Definition Types Examples 52 Off The physical state of a solution—solid, liquid, or gas—is typically the same as that of the solvent, as demonstrated by the examples in table 1. the components of a solution are dispersed on a molecular scale; that is, they consist of a mixture of separated molecules, atoms, and or ions. There are various ways to classify solutions based on different criteria, such as the nature of the solvent, the amount of solvent added, and the concentration of solute in the solution. here are some rephrased statements:. In this article we will discuss aqueous solution, types of solution, homogeneous solution, homogeneous solution examples and get detailed information including the different types, heterogeneous, examples, faqs and more here. Solutions are homogeneous mixtures of two or more substances having components that are uniformly distributed. in a solution, the material in the higher quantity is called the solvent, whereas the substance in the lesser quantity is called the solute.
Comments are closed.